By
Nick Paul Taylor Contributor
Dive Brief:
- Medtronic was ordered by a jury to pay $106.5 million for infringing a transcatheter aortic valve replacement (TAVR) patent.
- Colibri Heart Valve filed a lawsuit against Medtronic in 2019, accused it of infringing two patents related to the delivery and controlled release of replacement heart valves, according to court filings.
- The federal jury convened in California to cover the case sided with Colibri, concluding that three of Medtronic’s Evolut systems induce doctors to infringe a patent, it added.
Dive Insight:
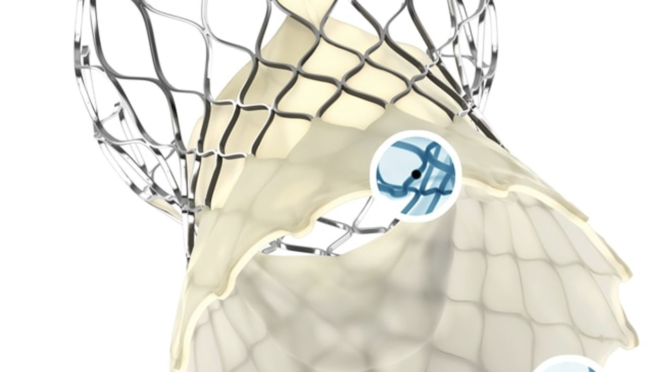
Colibri developed a “self-expanding heart valve device that includes cross-linked biological tissue and a delivery system that can be guided through a patient’s artery to the heart where it is positioned and used to replace diseased valves,” according to a lawsuit it filed in 2020 in a California federal court.
In its lawsuit, Colorado-based Colibri used identical language to describe Medtronic’s TAVR devices and argued the portfolio infringes its patents.
Colibri said that its CEO, Joseph Horn, gave a presentation to Medtronic employees in May 2014 under a nondisclosure agreement. Horn’s presentation covered Colibri’s heart valve products, delivery systems and methods. Medtronic brought a CoreValve TAVR system to market in 2014 and went on to release several upgrades. Colibri’s lawsuit notes similarities between Medtronic’s devices and its patents.
The jury ruled that Medtronic must pay Colibri $106.5 million for patent infringement.
“Medtronic strongly disagrees with the ruling and will continue to vigorously defend against these allegations at the appellate level,” a company spokesperson rote in an emailed statement. “In the meantime, Colibri’s patent has no impact on ongoing operations, as the patent expired in January 2022.”