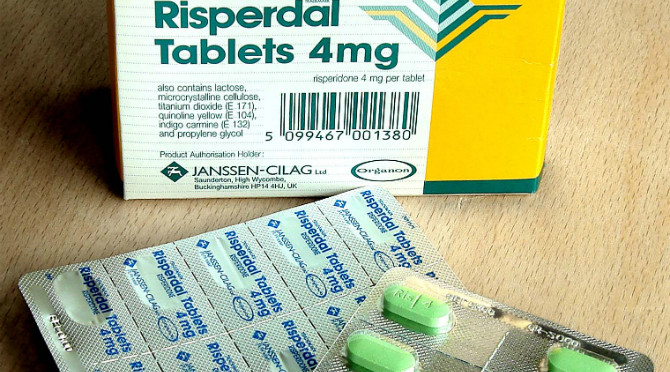
January 11, 2016 | By Tracy Staton
Johnson & Johnson ($JNJ) fell short Monday in its final effort to escape a Risperdal marketing penalty in South Carolina. The U.S. Supreme Court declined to take up J&J’s last appeal in the case, putting the company on the hook for a $124 million penalty.
J&J had cited the Eighth Amendment in arguing against the penalty, saying it qualified as an “excessive fine.” As Reuters notes, the U.S. Chamber of Commerce had backed the drugmaker in seeking Supreme Court review.
J&J’s Janssen unit has been fighting South Carolina’s deceptive trade practices court win since 2011, when a jury ordered the drugmaker to pay $327 million for Risperdal marketing violations. The company succeeded in lowering the judgment twice, first to $136 million and then, last year, to the final $124 million.
The lawsuit centered on promotional materials Janssen used to market the antipsychotic drug. Key to the case was a letter sent to South Carolina physicians, which overstated Risperdal’s benefits compared with other drugs in its class and downplayed side effects, the jury found. The trial court judge ordered Janssen to pay about $4,000 for each of the more than 7,000 letters mailed.
The original $327 million judgment dwarfed other similar rulings in drug-marketing lawsuits, including sizable decisions and settlements in other Risperdal-related litigation, but it fell far short of a $1.2 billion verdict in Arkansas. The Arkansas Supreme Court struck down that judgment in March 2014, and the company later negotiated a settlement of $7.5 million.
The South Carolina decision survived that state’s top court in a ruling last year, in which Justice John Kittredge backed the decision at trial, but lowered the $327 million penalty to $136 million.
In affirming the judgment against the company, Kittredge echoed the trial judge’s “profit-at-all-costs” characterization of Janssen’s marketing efforts. “Janssen’s desire for market share and increased sales knew no bounds, leading to its egregious violation of South Carolina law,” Kittredge wrote in the February 2015 ruling.
Janssen had argued that it did not intentionally deceive doctors with the now-notorious “Risperdal letter” that has featured in several state-court lawsuits. The drugmaker also contended that South Carolina’s attorney general didn’t prove patients were actually harmed by the drug. It was on that point that Kittredge lowered the judgment.
The “Risperdal letter” lawsuits compose only part of the mountain of litigation J&J has fought over the antipsychotic drug. The company agreed to pay $2.2 billion in a marketing settlement with the U.S. Justice Department and a group of states.
And the litigation isn’t over yet. The company now faces more than 1,000 lawsuits over Risperdal’s ability to trigger breast development in boys. J&J lost the first court battle last February, as a Philadelphia jury ordered J&J to pay almost $2.5 million to a young man who developed breasts while using Risperdal. In November, another jury awarded $1.75 million in a similar case.
Read Full Article – http://www.fiercepharma.com/story/time-jj-pay-124m-risperdal-case-scotus-deflects-final-appeal/2016-01-11